Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Formulation and Evaluation of Herbal Gel Utilizing Eucalyptus and Lemongrass Oils for Fungal Infection Treatment
Authors: Priya Sharma
DOI Link: https://doi.org/10.22214/ijraset.2024.63083
Certificate: View Certificate
Abstract
This study presents the formulation and evaluation of a novel herbal gel focus on treating fungal infections. The gel incorporates the therapeutic properties of eucalyptus and lemongrass oils, both known for their antifungal activities. The formulation process involved optimizing the concentration of active ingredients to maximize efficacy while ensuring safety and stability. Various parameters such as pH, viscosity, spreadability, and in vitro antifungal activity against common fungal pathogens were evaluated to assess the quality and performance of the gel. Results indicate that the developed herbal gel exhibits favorable physicochemical properties, including suitable pH and viscosity for topical application, as well as good spreadability. Moreover, the gel demonstrates significant antifungal activity against tested fungal strains, suggesting its potential as an effective treatment for fungal infections. Overall, this study highlights the promising prospects of herbal formulations containing eucalyptus and lemongrass oils in combating fungal infections, offering a natural alternative to conventional antifungal therapies. Further research and clinical trials are warranted to validate its efficacy and safety for widespread therapeutic use.
Introduction
I. INTRODUCTION
Applying gel directly to the affected area provides a advantage by facilitating a quicker release of medication directly to the site of infection[1]. A gel is a solid made up of at least two components, one of which, the polymer, forms a three-dimensional network in the medium of the other, the liquid, by means of covalent or non covalent bonding (chemical and physical gels, respectively)[2]. Herbs are used for fungal infections because they don't have the same limitations as synthetic treatments and are less harmful [3]. Herbs are potential to treat infectious diseases may open up new therapy options. Combination therapy shows stronger antimicrobial activity than using each antimicrobial agent alone. It broadens the spectrum of antimicrobial action, prevents the development of resistant strains, reduces toxicity, and achieves effective treatment with lower doses compared to synthetic drugs[4].Plants contain several compounds that are effective against different types of fungi, which is crucial for human health. Diverse bioactive secondary metabolites, including flavonoids, alkaloids, terpenoids, tannins, and saponins, are abundant in plants and have been shown to exhibit antifungal effects in vitro [5].
Eucalyptus globules a flowering plant species in the Myrtaceae family, is also referred to as blue gum or southern blue gum found in Americas , Europe , Africa , Middle east , Indian subcontinent [6] has chemical composition of 1,8-cineole, limonene, p-cymene, γ-terpinene, α-pinene, α-terpineol, camphene, linalool, and ocimene [7] in which 1,8-cineole (also known as eucalyptol) is major component[8].1,8-cineole could potentially target the enzymes glucosamine-6-phosphate (GlcN-6-P) synthase, UDP-glycosyltransferase (UGT), and chitin synthase (CHS), which are critical for fungal cell wall construction[9-10]. Essential oil of eucalyptus is complex combination of various monoterpenes and sesquiterpenes, as well as aromatic phenols, oxides, ethers, alcohols, esters, aldehydes, and ketones, including 1,8-cineole , citronellal, citronellol, citronellyl acetate, p-cymene, eucamalol, limonene, linalool, α-pinene, γ-terpinene, α-terpineol, and aromadendrene, make up eucalyptus oil[11].
Cymbopogon citrates also known as lemon grass is an aromatic perennial tall grass with rhizomes and densely tufted fibrous root. It has short underground stems with ringed segments, coarse, green slightly leathery leaves in dense clusters .[12] The composition of its essential oil varies depending on when the grass is harvested. When lemongrass is harvested 5.5 months after planting, its essential oil comprises 44 molecules, making up 98.64% of the oil's composition. However, when harvested at 6.5 months, only 15 chemical components account for 98.62% of the oil. Interestingly, the concentration of geranial in lemongrass oil increases from 37.58% to 45.95% when the leaves are harvested at 5.5 and 6.5 months after planting, respectively. Extending the harvest period to 7.5 months results in a slight decrease in essential oil content to 42.95%.
Lemongrass leaves with the highest concentration of essential oil (2.45%) exhibit strong antibacterial qualities.[13]. A significant portion of citral found in lemon grass essential oil is utilized as a raw material to produce ionone, vitamin A, beta carotene, and other compounds.
Antimicrobial activity of LGO has been studied against several pathogenic fungi [14].The different compositions of Cymbopogon species found to be very potential for antifungal activities[15]. The major identified compounds in LEO were geranial (45%), neral (20%) undececanone (20%), cuparene (11%) and geranial acetate (6%) . In silico molecular docking studies revealed that geranial and geraniol from LEO act as effective ligands. These ligands docked with fungal cell wall enzymes like RS (possibly ribosomal protein synthesis), RibD (riboflavin biosynthesis protein), and DBPSG (possibly an enzyme involved in the biosynthesis of cell wall components). Geranial and geraniol from LEO and PEO likely inhibit the activity of fungal enzymes such as RS, RibD, and DBPSG. By interfering with these enzymes, essential processes like protein synthesis and cell wall biosynthesis are disrupted, leading to impaired fungal growth and viability. Overall, the mechanism of action of lemon grass essential oil against fungal infections involves the inhibition of key fungal enzymes involved in cell wall biosynthesis, leading to impaired fungal growth and survival[16].
II. MATERIALS AND METHODS
Essential oil: pure essential oil of eucalyptus globules and Cymbophogan citrus Other materials- methyl cellulose, polypropylene glycol 4000, sodium benzoate, triethanolamaine, glycerine
Preperation of gel containing selected essential oil 5 ml of distilled water was measured and placed in a clean, dry beaker. The water was heated on a heating mantle until it reached temperature above 80 degrees Celsius. The temperature was monitored with a thermometer. Once the water reached the desired temperature, it was allowed to cool to room temperature. Methyl cellulose was added to the distilled water while stirring continuously until it was uniformly mixed and a gel base was formed.A water bath was prepared, and enough water was added to cover the bottom of a beaker containing 10 ml of distilled water.The required quantity of sodium benzoate was added to the 10 ml of distilled water in the beaker, and it was placed on the water bath. After 5 minutes of heating, the appropriate amount of Polyethylene Glycol (PEG) was added to the sodium benzoate solution. It was stirred until the PEG was fully dissolved. The specified essential oils were added to the preservative solution containing sodium benzoate and PEG. The mixture was stirred thoroughly to ensure uniform mixing. The preservative solution with essential oils was slowly added to the gel base prepared earlier. The mixture was stirred gently but thoroughly to ensure even distribution of the preservative solution within the gel base. pH of the final product was measured. Triethanolamine was added dropwise to the gel base while stirring continuously. The addition of triethanolamine was continued until the pH reached the desired neutral level. The prepared sodium benzoate gel with essential oils was transferred to air tight container. The product was stored in a cool, dry place away from direct sunlight.
Evaluation of gel preperations
Physical Evaluation Physical parameters such as color and appearance were checked.
Measurement of pH About 1 g gel was accurately weighed and dispersed in 100 ml purified water. The pH of the dispersion was measured using digital pH meter. The measurements of pH were done in triplicate and average values were calculated[17].
Viscosity: Viscosity of herbal gel was determined by using Brookfield rotational viscometer at 5, 10 20, 30 and 50 rpm using spindle no.64. Each reading was taken after equilibrium of the sample at the end of two minutes. The viscosity determination of samples was repeated three times[18].
Spreadability: The parallel-plate method is the approach most frequently used to determine spreadability. One gram of the sample, which was prepared 48 hours before to the test, is sandwiched between two 20 by 20-cm glass plates for the parallel-plate measurement. For one minute, a 125 g weight (between 50 and 500 g) is placed on top. Next, the sample's diameter between the plates is measured. A test sample of a specific mass is put on a glass plate for the experiment, and it is covered with another plate that has a wooden block attached to it. For a while, a weight is applied to the upper plate. The weight is then taken out, the holding agent is fastened to the wooden block, and lower plates to fully separate is calculated[19].
Spreadability is determined by the formula:
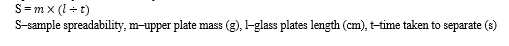
|
Drug |
F1 |
F2 |
F3 |
|
Eucalyptus globules essential oil (ml) |
1.25 |
1.5 |
1.75 |
|
Cymbopogon citrates essential oil (ml) |
1 |
1 |
1 |
|
Methyl cellulose (g) |
0.75 |
1 |
1.25 |
|
Polyethylene glycol 4000 (g) |
2 |
2 |
2 |
|
Sodium benzoate (g) |
0.5 |
0.5 |
0.5 |
|
Glycerine (ml) |
2 |
2 |
2 |
|
Triethanoalamine |
qs |
qs |
qs |
|
Distilled water |
qs |
qs |
qs |
Table no 1 Indegriedients used in formulation
- Physical Evaluation: Colour and appearance of the formulation is checked and is as mentioned below in table
Table no. 2 Physical evaluation
|
Test |
Result |
|
Colour |
white |
|
Apearance |
gel |
2. Measurement of ph: ph was observed according to gel ph which is 7-9.
Table no.3 Measurement of ph results
|
S no |
Formulation Code |
pH |
|
|
1 |
F1 |
7.8 |
|
|
2 |
F2 |
7.1 |
|
|
3 |
F3 |
8 |
|
3. Viscosity: The viscosity of the samples was measured and analyzed.
Table no. 4 Viscosity of gel
|
S no |
Formulation |
Cp (centipoise) |
|
1 |
F1 |
2020 |
|
2 |
F2 |
2056 |
|
3 |
F3 |
3743 |
4. Spreadability: The spreadability of the samples was assessed and calculated by using formula.
Table no. 5 Spreadability of gel
|
S no |
Formulation |
Spreadability (gm/cm/sec) |
|
1 |
F1 |
25.4 |
|
2 |
F2 |
26.99 |
|
3 |
F3 |
19.24 |
5. Fourier-transform infrared spectroscopy
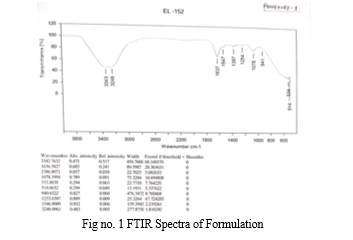
The FTIR spectrum of Cymbopogon citrates (lemongrass) oil revealed several prominent peaks at wavenumbers of 2970, 2921, 2862, 1674, 1637, 1383, and 1127 cm^-1[20] .These peaks indicate the presence of various functional groups within the oil.
The presence of a peak at 1547 cm^-1 suggests the presence of C=O stretching vibrations, indicative of amide groups, while the peak at 1637 cm^-1 may correspond to additional functional groups such as carbonyl compounds. Hence Cymbopogon citrates oil contains a complex mixture of organic compounds, including alkanes, amides, phenols, and alcohols, which may contribute to its various biological activities and potential applications in pharmaceuticals.
In the FT-IR spectrum of eucalyptus , a broad peak at 1457 cm^-1 attributed to C-H bending due to alkanes, and peaks around 1214 and 1079 cm^-1 indicating esters and alcohols, respectively, confirming the presence of aromatic compounds, alcohols, phenols, alkanes, alkynes, and amines in Eucalyptus oil[21].In comparison to previous studies on Eucalyptus oil, our FTIR analysis revealed similar functional groups present in the sample. Specifically, our results showed distinct peaks at 1254, and 1078 cm^-1, which correspond to specific vibrational modes associated with certain chemical functionalities within the oil and they confirm presence of esters and alcohols, respectively, confirming the presence of these functional groups in the Eucalyptus oil.
pure methylcellulose
vibration of hydroxyl group ν(O-H).
Pure methylcellulose had an absorption band at 3468.28 cm-1 related to stretching vibration of hydroxyl group ν(O-H)[22].In our results, the absorption band at 3443 cm^-1 indicates the stretching vibration of the hydroxyl group ν(O-H), consistent with pure methylcellulose characteristics. Reported peaks of polyethylene glycol 4000 is 1735, 1349, and 1096 cm^-1[23].Our results indicate peaks at 1387 and 941 cm^-1, suggesting specific vibrational modes in the FTIR spectrum of PEG 400, possibly associated with functional groups such as alkane stretching and C-O stretching, respectively, in comparison to the reported peaks.
Conclusion
On the basis of the previous findings, it can be concluded that the formulation and evaluation of a herbal gel utilizing eucalyptus and lemongrass oil for the treatment of fungal infections have been successfully accomplished. Through evaluation of key physical attributes such as pH, viscosity, and spreadability, as well as FTIR analysis confirming chemical stability, the herbal gel has demonstrated optimal qualities essential for effective topical application. This study concludes that when lemongrass and eucalyptus oils are combined and incorporated into a gel, there is no interaction between the drug and polymer and it can effectively and profoundly treat many superficial fungal infections.
References
[1] Misal G, Dixit G, Gulkari V. Formulation and evaluation of herbal gel. [2] Almdal K, Dyre J, Hvidt S, Kramer O. Towards a phenomenological definition of the term ‘gel’. Polymer gels and networks. 1993 Jan 1;1(1):5-17. [3] Khairy WA, [et al.]. Prevalence and predictors of self-medication with antifungal drugs and herbal products among university students: a cross-sectional study from Egypt. Risk ManagHealthc Policy. 2021;14:2191-2200. [4] Herman A, Herman AP. Herbal products and their active constituents used alone and in combination with antifungal drugs against drug-resistant Candida sp. Antibiotics. 2021;10(6):655. [5] Arif T, [et al.]. Natural products–antifungal agents derived from plants. J Asian Nat Prod Res. 2009;11(7):621-638. [6] Sellers CH. Eucalyptus: Its History, Growth, and Utilization. A.J. Johnston; 1910.p. 13.OCLC 903889267. [7] Zhou LJ, Huang LJ, Yang ZR, Bai LH. Optimization of supercritical CO2 extraction conditions for essential oil from Eucalyptus grandis - Eucalyptus urophylla using Box-Behnken design-response surface methodology. J Sichuan Univ (Nat Sci Ed). 2014;51:1319–1324. [8] Cimanga K, Kambu K, Tona L, Apers S, De Bruyne T, Hermans N, Totté J, Pieters L, Vlietinck AJ. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo.J Ethnopharmacol. 2002;79:213–220. [9] Martin G, Zhao J, An M, Agboola S. Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem. 2010;119(2):731-737. doi:10.1016/j.foodchem.2009.07.021. [10] Singh K, Deepa N, Chauhan S, Tandon S, Verma RS, Singh A. Antifungal action of 1,8 cineole, a major component of Eucalyptus globulus essential oil against –Alternaria tenuissima via overproduction of reactive oxygen species and downregulation of virulence and ergosterol biosynthetic genes. Ind Crops Prod. 2024;214:118580. [11] Abbasi, Naser, et al. \"Extraction and phytoanalysis of chemical compounds of Eucalyptus globulus leaf native to Dehloran, Ilam province, Iran by HS-SPME and GC-MS.\" Advances in animal and veterinary sciences 8.6 (2020): 647-652. [12] 12.Nambiar, V. S., &Matela, H. (2012). Potential functions of lemon grass (Cymbopogoncitratus) in health and disease. International Journal of Pharmaceutical and Biological Archives, 3(5), 1035-1043. [13] 13.Kumoro, A. C., et al. (2021). A brief review on the characteristics, extraction and potential industrial applications of citronella grass (Cymbopogonnardus) and lemongrass (Cymbopogoncitratus) essential oils.IOP Conference Series: Materials Science and Engineering, 1053(1). IOP Publishing. [14] Tyagi AK, Malik A. In situ SEM, TEM and AFM studies of the antimicrobial activity of lemon grass oil in liquid and vapour phase against Candida albicans. Micron. 2010;41(7):797-805. [15] Devi MA, Sahoo D, Singh TB, Rajashekar Y. Antifungal activity and volatile organic compounds analysis of essential oils from Cymbopogon species using solid-phase microextraction-gas chromatography-mass spectrometry. J Agric Food Res. 2021;3:100110. [16] Sharma AD, Kaur I, Chauhan A. Anti-aspergillosis and anti-mucormycosis potential of essential oils from two Cymbopogon spp. targeting riboflavin synthesis pathway. Phytomed Plus. 2023;3(2):100440. [17] Vandana D, Pawar S. Formulation and evaluation of topical herbal gel containing inclusion complex of curcumin. Asian J Pharm Clin Res. 2019;12(9):196-201. [18] Jadhav VD, et al. Formulation and evaluation of herbal gel containing leaf extract of Tridax Procumbens. J Pharm Biosci. 2015;3:65-72. [19] Alexander I, Krasnyuk II. Dermatologic gels spreadability measuring methods comparative study. Int J App Pharm. 2022;14(1):164-168. [20] Kumar A, Dev K, Sourirajan A. Essential Oils of Rosmarinus officinalis L., Cymbopogon citratus (DC.) Stapf., and the phyto-compounds, delta-carene and alpha-pinene mediate cell cycle arrest at G2/M transition in budding yeast Saccharomyces cerevisiae. South African Journal of Botany. 2021;141:296-305. [21] Sharma AD, Farmaha M, Kaur I, Singh N. Phytochemical analysis using GC-FID, FPLC fingerprinting, antioxidant, antimicrobial, anti-inflammatory activities analysis of traditionally used Eucalyptus globulus essential oil. Drug Analytical Research. 2021 Jun 30;5(1):26-38. [22] Nadour M, Daoud F, Ouradi A, Benaboura A. Effects of Methylcellulose on the Properties and Morphology of Polysulfone Membranes Prepared by Phase Inversion. Materials Research. 2017;20. [23] Khan BA, Ahmad N, Alqahtani A, Baloch R, Rehman AU, Khan MK. Formulation development of pharmaceutical nanoemulgel for transdermal delivery of feboxostat: Physical characterization and in vivo evaluation. European Journal of Pharmaceutical Sciences. 2024;195:106665. doi:10.1016/j.ejps.2024.106665.
Copyright
Copyright © 2024 Priya Sharma. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63083
Publish Date : 2024-06-03
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

